Metea alumna Myra Bajwa gives insight on her experience with a COVID-19 vaccine trial
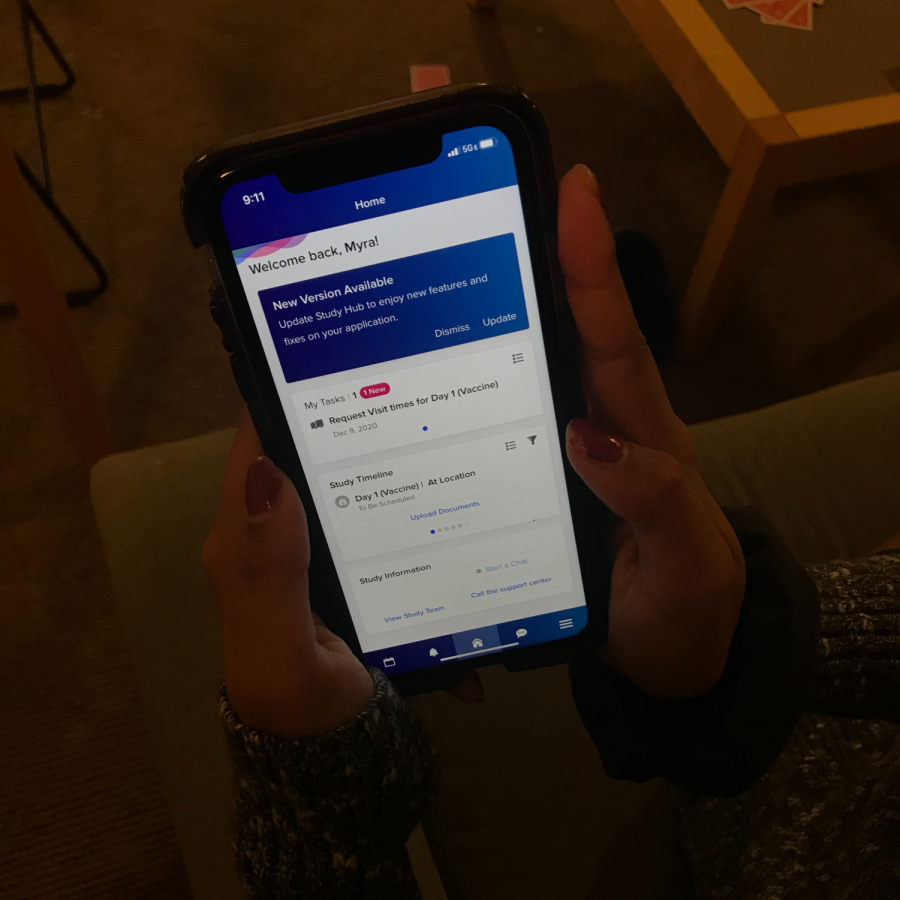
Myra Bajwa uses an app to update her health status.
January 14, 2021
After almost a year since the first COVID-19 case was announced in the United States, vaccines are starting to roll out to essential workers and the elderly. Johnson & Johnson is one of the many corporations that are developing and testing their own COVID-19 vaccine. In order to research how effective the vaccine is, Johnson & Johnson has started vaccine trials for those who want to participate. Myra Bajwa, who graduated last May, is amongst the students at the University of Chicago to participate in this trial.
“A mass email was sent from the University of Chicago Medicine because we have a really big hospital here,” Bajwa said. “They do a ton of research. And so, there are actually a couple of trials going on on campus. The email said the trial needed people of different racial backgrounds, different ages, different genders, and all that, so if you’re interested, you can sign up to be on the list. So I signed up to be on the list, and then they contacted me and asked when I was available for the first dose.”
On-campus college students are more likely to get COVID-19 than those who are attending from home. Conducting a vaccine trial on those who are more prone to the virus will be more efficient in the research process, as it tests the effectiveness of the vaccine against it.
“I am going to class in person, I see friends on campus,” Bajwa said. “I do socialize more than the average person, who’s not on a college campus if we’re being honest with ourselves. So if they really want to know where there is a high chance to get COVID-19, I think college students are a really good outlet for that.”
Not all vaccine trials are the same. In Bajwa’s case, the trial was a double-blind study in which neither the one receiving the vaccine nor the person giving it knows who is getting the real shot. Double-blind studies are good for preventing bias or the placebo effect from happening.
“I went in early December and I got the shot, whether that was a placebo or the actual vaccine, I don’t know,” Bajwa said. “There are a couple of checkups, and each visit they take your blood. Every Monday and Thursday on my little app I have to report whether or not I’m having COVID-19 symptoms. It’s really easy. It’s just one tap of a button, and I’m done- but I get compensated for my time at each visit. So it’s $200 per visit, which has been nice.”
The trial resembled a hospital check-up, but with fewer healthcare workers, resulting in a longer waiting time for Bajwa. The outcome of receiving any vaccine is different for all.
“They honestly didn’t have that many centers,” Bajwa said. “I will say, the first visit was kind of a long visit. I was there for three hours because they checked everything from weight, height, pregnancy test, tons of blood tests, and so on. You can’t eat if you have a mask on, so they just kept taking blood, and I was kind of woozy when I left. The shot itself felt like a flu shot. My arm was kind of sore, so I didn’t sleep on that arm. But other than that, it was fine. No headaches, no fever, nothing like that.”
Research testees and trials, such as this one, can be nerve-wracking for some people. However, Bajwa embraced the fact that she is a young and healthy college student, she would not be as likely to have severe drawbacks from the experiment.
“I felt fine- until I sat there and realized what I got myself into,” Bajwa said. “I mean, obviously one of the drawbacks is, if I got a placebo, I can’t get the real vaccine until this two-year study is over. But on the flip side, as a young and healthy 19-year-old, I probably wasn’t going to get it for a while because I’m at the very end of that line. I know a lot of people my age have had issues with COVID-19, but I feel like if anyone’s gonna tries out a placebo I think it’s okay if it’s me so I think I’ll be okay.”